
ACILA® offers endotoxins (lipopolysaccharides or LPSs) for various applications, including Limulus Amebocyte Lysate Tests (LAL Test) and Pyrogen Tests. They can also be used as bioindicators for the validation of depyrogenation processes or ultrafiltration. The endotoxins are from different species of bacteria and therefore contain different amounts of lipid A, which means that their activity also varies. Our endotoxins are highly purified and do not contain any fillers, opposite to most endotoxin preparations.
The Pharmacopoeias (Europe, USA, Japan), the World Health Organisation (WHO) and the Food and Drug Administration (FDA) differentiate between Reference Standard Endotoxin (RSE) and Control Standard Endotoxin (CSE). Due to the harmonization of Limulus Amebocyte Lysate Test (LAL Test) in the three pharmacopoeias, there are only activity-identical endotoxin units since 1997:
1 I.U. (I.E) (International Unit) = 1 E.U. (Endotoxin Unit, FDA) = 1 USP Unit
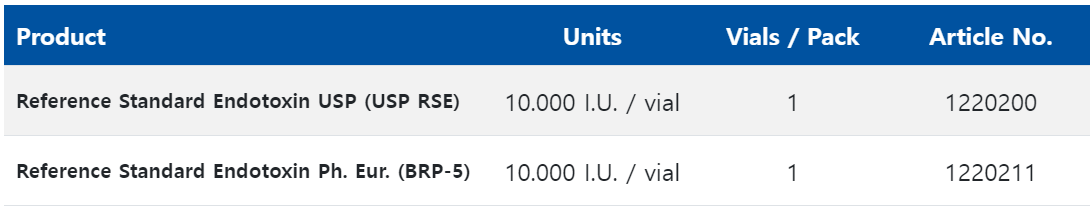
These Reference Standard Endotoxins are, however, still found with different product names and with different units depending on their origin. The International Standard of the WHO and the EC-6 of the FDA are available only for certain reference laboratories or producers of Limulus lysate, while the Reference Standard Endotoxins of USP and EP can be purchased from ACILA.
(주)태평양과학은 개인정보보호법에 따라 정보주체의 개인정보를 보호하고 이와 관련한 고충을 신속하고 원활하게 처리할 수 있도록 하기 위하여 다음과 같이 개인정보 처리지침을 수립·공개합니다.
(주)태평양과학은 개인정보를 다음의 목적을 위해 처리합니다. 처리한 개인정보는 다음의 목적이외의 용도로는 사용되지 않으며 이용 목적이 변경될 시에는 사전 동의를 구할 예정입니다.
가. 재화 또는 서비스 제공상담서비스 제공
콘텐츠 제공 등을 목적으로 개인정보를 처리합니다.
(주)태평양과학은 법령에 따른 개인정보 보유·이용기간 또는 정보주체로부터 개인정보를 수집 시에 동의 받은 개인정보 보유·이용기간 내에서 개인정보를 처리·보유합니다.
1. 사업신청: 연 사업종료 시
2. 자원봉사 신청 : 연 사업종료 시
(주)태평양과학은 정보주체의 동의, 법률의 특별한 규정 등 개인정보 보호법 제 17조 및 제18조에 해당하는 경우에만 개인정보를 제3자에게 제공합니다.
(주)태평양과학은 원활한 개인정보 업무처리를 위하여 다음과 같이 개인정보 처리업무를 위탁하고 있습니다.
- 위탁받는 자 (수탁자) : 솔루웨이
- 위탁하는 업무의 내용 : 홈페이지 시스템 운영 및 유지보수
이용자는 개인정보주체로서 다음과 같은 권리를 행사할 수 있습니다.
1. 정보주체는 (주)태평양과학은에 대해 언제든지 다음 각 호의 개인정보 보호 관련 권리를 행사할 수 있습니다.
1) 개인정보 열람요구
2) 오류 등이 있을 경우 정정 요구
3) 삭제요구
4) 처리정지 요구
2. 제1항에 따른 권리 행사는 (주)태평양과학에 대해 개인정보 보호법 시행령 제41조 제1항에 따라 서면, 전자우편, 모사전송(FAX) 등을 통하여 하실 수 있으며 (주)태평양과학은 이에 대해 지체 없이 조치하겠습니다.
3. 정보주체가 개인정보의 오류 등에 대한 정정 또는 삭제를 요구한 경우에는 (주)태평양과학은 정정 또는 삭제를 완료할 때까지 당해 개인정보를 이용하거나 제공하지 않습니다.
4. 제1항에 따른 권리 행사는 정보주체의 법정대리인이나 위임을 받은 자 등 대리인을 통하여 하실 수 있습니다. 이 경우 개인정보 보호법 시행규칙 별지 제11호 서식에 따른 위임장을 제출하셔야 합니다.
5. 개인정보 열람 및 처리정지 요구는 개인정보보호법 제35조 제5항, 제37조 제2항에 의하여 정보주체의 권리가 제한될 수 있습니다.
6. 개인정보의 정정 및 삭제 요구는 다른 법령에서 그 개인정보가 수집 대상으로 명시되어 있는 경우에는 그 삭제를 요구할 수 없습니다.
7. (주)태평양과학은 정보주체 권리에 따른 열람의 요구, 정정·삭제의 요구, 처리정지의 요구 시 열람 등 요구를 한 자가 본인이거나 정당한 대리인인지를 확인합니다.
(주)태평양과학은 다음의 개인정보 항목을 처리하고 있습니다.
재화 및 서비스제공
1. 사업신청: 기관/단체명, 기관주소, 이름 및 직책, 전화번호(사무실, 휴대전화), 전자우편 주소
2. 자원봉사 신청: 이름, 나이, 전화번호, 소속(학교 및 기관), 전자우편 주소, 경력사항
3. 인터넷 서비스 이용과정에서 아래 개인정보 항목이 자동으로 생성되어 수집될 수 있습니다. IP주소, 쿠키, MAC주소, 서비스이용기록, 방문기록, 불량이용기록 등
(주)태평양과학은 다음의 개인정보 항목을 처리하고 있습니다.
재화 및 서비스제공
1. (주)태평양과학은 원칙적으로 개인정보 처리목적 달성 등 개인정보가 불필요하게 되었을 때에는 지체 없이 해당 개인정보를 파기합니다.
2. 정보주체로부터 동의 받은 개인정보 보유기간이 경과하거나 처리목적이 달성되었음에도 불구하고 다른 법령에 따라 개인정보를 계속 보존하여야 하는 경우에는, 해당 개인정보를 별도의 데이터베이스(DB)로 옮기거나 보관 장소를 달리하여 보존합니다.
3. 개인정보 파기의 절차 및 방법은 다음과 같습니다.
1) 파기절차: (주)태평양과학은 파기하여야 하는 개인정보에 대해 개인정보 파기계획을 수립하여 파기합니다. (주)태평양과학은 파기사유가 발생한 개인정보를 선정하고, (주)태평양과학의 개인정보 보호책임자의 승인을 받아 개인정보를 파기합니다.
2) 파기방법: (주)태평양과학은 전자적 파일 형태의 정보는 기록을 재생할 수 없도록 파기하며, 종이 문서에 기록·저장된 개인정보는 분쇄기로 분쇄하거나 소각하여 파기합니다.
(주)태평양과학은 개인정보의 안전성 확보를 위해 다음과 같은 조치를 취하고 있습니다.
1. 관리적 조치 : 내부관리계획 수립·시행, 정기적 직원 교육 등
2. 기술적 조치 : 개인정보처리시스템 등의 접근권한 관리, 개인정보 접속기록관리시스템 운영, 고유식별정보 등의 암호화, 보안프로그램 설치
3. 물리적 조치 : 자료보관실 등의 접근통제
(주)태평양과학은 개인정보보호법 제29조에 따라 다음과 같이 안전성 확보에 필요한 기술적/관리적 및 물리적 조치를 하고 있습니다.
1. (주)태평양과학은 이용자에게 개별적인 맞춤서비스를 제공하기 위해 이용정보를 저장하고 수시로 불러오는 '쿠키(cookie)'를 사용합니다.
2. 쿠키는 웹사이트를 운영하는데 이용되는 서버(http)가 이용자의 컴퓨터 브라우저에게 보내는 소량의 정보이며 이용자의 PC컴퓨터 내의 하드디스크에 저장되기도 합니다.
1) 쿠키의 사용목적: 이용자가 방문한 각 서비스와 웹 사이트들에 대한 방문 및 이용형태, 인기검색어, 보안접속 여부 등을 파악하여 이용자에게 최적화된 정보제공을 위해 사용됩니다.
2) 쿠키의 설치·운영 및 거부: 웹브라우저 상단의 도구→인터넷옵션→개인정보 메뉴의 옵션 설정을 통해 쿠키 저장을 거부할 수 있습니다.
3) 쿠키 저장을 거부할 경우 맞춤형 서비스 이용에 어려움이 발생할 수 있습니다.
1. (주)태평양과학은 개인정보 처리에 관한 업무를 총괄해서 책임지고, 개인정보 처리와 관련한 정보주체의 불만처리 및 피해구제 등을 위하여 아래와 같이 개인정보 보호책임자를 지정하고 있습니다.
▶ 개인정보 보호책임자: ***
- 전화번호: 031-750-9190
- 팩스번호: 031-750-9191
2. 정보주체께서는 (주)태평양과학의 서비스(또는 사업)를 이용하시면서 발생한 모든 개인정보 보호 관련 문의, 불만처리, 피해구제 등에 관한 사항을 개인정보 보호책임자 및 담당부서로 문의하실 수 있습니다. (주)태평양과학은 정보주체의 문의에 대해 지체 없이 답변 및 처리해드릴 것입니다.
정보주체는 개인정보 보호법 제35조에 따른 개인정보의 열람 청구를 개인정보 보호 담당부서로 할 수 있습니다. (주)태평양과학은 정보주체의 개인정보 열람청구가 신속하게 처리되도록 노력하겠습니다.
정보주체께서는 제1항의 열람청구 접수·처리부서 이외에, 행정안전부의 ‘개인정보보호 종합지원 포털’ 웹사이트(www.privacy.go.kr)를 통하여서도 개인정보 열람청구를 하실 수 있습니다.
▶ 행정안전부 개인정보보호 종합지원 포털 → 개인정보 민원 → 개인정보 열람등 요구 (공공아이핀을 통한 실명인증 필요)
정보주체는 아래의 기관에 대해 개인정보 침해에 대한 피해구제, 상담 등을 문의하실 수 있습니다.
※ 아래의 기관은 (주)태평양과학와는 별개의 기관으로서, (주)태평양과학의 자체적인 개인정보 불만처리, 피해구제 결과에 만족하지 못하시거나 보다 자세한 도움이 필요하시면 문의하여 주시기 바랍니다.
□ 개인정보 침해신고센터 (한국인터넷진흥원 운영)
- 소관업무 : 개인정보 침해사실 신고, 상담 신청
- 홈페이지 : privacy.kisa.or.kr
- 전화 : (국번없이) 118
- 주소 : (05717) 서울특별시 송파구 중대로 135 한국인터넷진흥원 개인정보침해신고센터
□ 개인정보 분쟁조정위원회
- 소관업무 : 개인정보 분쟁조정신청, 집단분쟁조정 (민사적 해결)
- 홈페이지 : www.kopico.go.kr
- 전화 : (국번없이) 1833-6972
- 주소 : (03171) 서울특별시 종로구 세종대로 209 정부서울청사 4층
□ 검찰청 사이버범죄수사단
- 소관업무 : 개인정보 침해 관련 형사사건 문의 및 신고
- 홈페이지 : www.spo.go.kr
- 전화 : 02-3480-3573
□ 검찰청 사이버안전국
- 소관업무 : 개인정보 사이버범죄 신고 및 상담고
- 홈페이지 : cyberbureau.police.go.kr
- 전화 : (국번없이) 182
(주)태평양과학은 영상정보처리기기를 설치·운영하고 있지 않습니다.
이 개인정보 처리방침은 2022. 07. 01 적용됩니다.
(주)태평양과학 이용약관 내용이 들어갑니다.
(주)태평양과학은 개인정보를 다음의 목적을 위해 처리합니다. 처리한 개인정보는 다음의 목적이외의 용도로는 사용되지 않으며 이용 목적이 변경될 시에는 사전 동의를 구할 예정입니다.
가. 재화 또는 서비스 제공상담서비스 제공
콘텐츠 제공 등을 목적으로 개인정보를 처리합니다.
(주)태평양과학은 개인정보를 다음의 목적을 위해 처리합니다. 처리한 개인정보는 다음의 목적이외의 용도로는 사용되지 않으며 이용 목적이 변경될 시에는 사전 동의를 구할 예정입니다.
가. 재화 또는 서비스 제공상담서비스 제공
콘텐츠 제공 등을 목적으로 개인정보를 처리합니다.
본 웹 사이트에 게시된 이메일 주소가 전자우편 수집 프로그램이나 그 밖의 기술적 장치를 이용하여 무단으로 수집되는 것을 거부하며 이를 위반 시 정보통신망법에 의해 형사 처벌됨을 유념하시기 바랍니다.